The information presented on this page may be dated. It may refer to situations which have changed or people who are no longer affiliated with the university. It is archived as part of Mississippi State University's history.
The knee is the largest and strongest joint in the body. But once damage sets in-from lesions caused by traumatic injury or gradual degeneration from osteoarthritis-this once mighty joint can bring an active body to a painful, screeching halt.
Since the late 1980s, microfracture has been the most common treatment to repair damaged cartilage, with U.S. surgeons performing over 100,000 of these procedures annually in recent years. It works by creating tiny fractures in the bone that underlies cartilage in the knee joint, which induce the bone marrow to produce fibrocartilage.
Unfortunately, the results are temporary because fibrocartilage, which doesn't naturally exist in the joint, does not absorb impact or last as long as the knee's original hyaline cartilage. A MAFES scientist is working toward solutions to enhance this procedure by creating a drug delivery system that would help the joint reproduce its normal-and more durable-hyaline cartilage.
In 2020, Dr. Steven Elder, MAFES scientist and professor in the Department of Agricultural and Biological Engineering, began exploring these possibilities. "I've spent years researching cartilage tissue and studying ways to regenerate that tissue to prevent or treat osteoarthritis," Elder said. "A few years back, I had the idea of building a bioactive and biodegradable cell scaffold. The structure would deliver kartogenin, a drug that tells stem cells in the bone marrow to produce normal cartilage. It would work by allowing a slow release of the drug into the knee and would dissolve when the job is done."
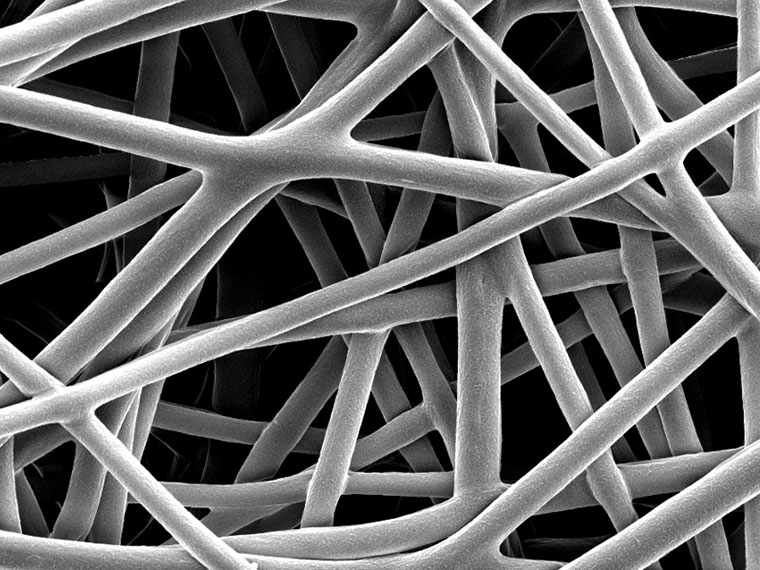
Elder joined colleagues Dr. Matthew Ross, professor in the College of Veterinary Medicine, and Dr. Sean Stokes, assistant professor in the Department of Chemistry, along with biomedical and biochemistry undergraduates, to find a solution. In the early days of the study, Elder and his colleagues and students investigated the viability of bioprinted biological materials created by a specialized 3-D printer to create the scaffolds. But the results were not exactly what they had hoped for. "The material was bulky and rigid and not ideal to place inside of a knee," Elder said. "So we tried a slightly different approach using technology called electrospinning."
With electrospinning, the same materials that went into the bioprinter produced a flexible sheet that would mold to the shape of the joint. When placed in a saline solution, the electrospun scaffolds lasted six months, about the amount of time the body takes to absorb kartogenin and produce new cartilage from bone marrow. The results of this study were published last year in the academic journal Molecules. The scientists' research is a preliminary step toward a future where this technology might be used in the operating room, but even at this early stage, the research has potential. Elder and his colleagues are currently studying a microparticle-based, injectable kartogenin delivery system that could slow or possibly halt the progress of osteoarthritis.
"Unfortunately, there are limited treatment options for patients suffering from cartilage degeneration," Elder said. "Cartilage transplant procedures have the best long-term results, but they come with some significant drawbacks and limitations. We will continue to search for solutions that we hope will one day improve the lives of people suffering from knee pain."
This research is funded by the Mississippi Agricultural and Forestry Experiment Station through a Strategic Research Initiative grant. The Undergraduate Research Scholars student, Robert Lawson, was funded by the MSU Shackouls Honors College and the Mississippi Agricultural and Forestry Experiment Station.
I've spent years researching cartilage tissue and studying ways to regenerate that tissue... Bioactive and biodegradable cell scaffold... Would deliver kartogenin, a drug that tells stem cells in the bone marrow to produce normal cartilage.
DR. STEVEN ELDER
Behind the Science

Steven Elder
Professor
Education: : B.S., Biomedical Engineering, Duke University; M.S., Biomedical Engineering, University of Iowa; Ph.D., Biomedical Engineering, University of Michigan
Years At MSU: 24
Focus: Orthopedics, with a focus on articular cartilage
Passion At Work: We are pursuing this research in the hopes that it will lead to a new treatment for cartilage injuries that will prevent or drastically delay the onset of